Introducing the gizmos collision theory answer key, an indispensable tool for understanding the fundamental principles governing molecular interactions. This comprehensive guide unravels the intricacies of collision theory, empowering learners to master this crucial concept in chemistry.
Delving into the realm of molecular motion, this exploration illuminates the factors that influence the frequency and outcomes of collisions between molecules. Through a series of interactive simulations and data analysis exercises, learners gain hands-on experience in applying collision theory to real-world scenarios.
Gizmo’s Collision Theory Simulation
The Gizmo’s Collision Theory simulation is a virtual laboratory that allows students to explore the relationship between temperature and the rate of collisions between gas particles.
The simulation includes several variables that can be manipulated, including the temperature of the gas, the number of gas particles, and the size of the gas particles.
To use the simulation, students first select the desired variables. They then click the “Start” button to begin the simulation. The simulation will run for a specified amount of time, and the results will be displayed in a graph.
Collision Theory
Collision theory is a theory that explains the rates of chemical reactions. The theory states that the rate of a chemical reaction is proportional to the number of collisions between the reactants.
The rate of collisions is affected by several factors, including the temperature of the reactants, the concentration of the reactants, and the activation energy of the reaction.
The temperature of the reactants has a significant effect on the rate of collisions. As the temperature increases, the average kinetic energy of the reactants increases. This increase in kinetic energy leads to an increase in the number of collisions between the reactants.
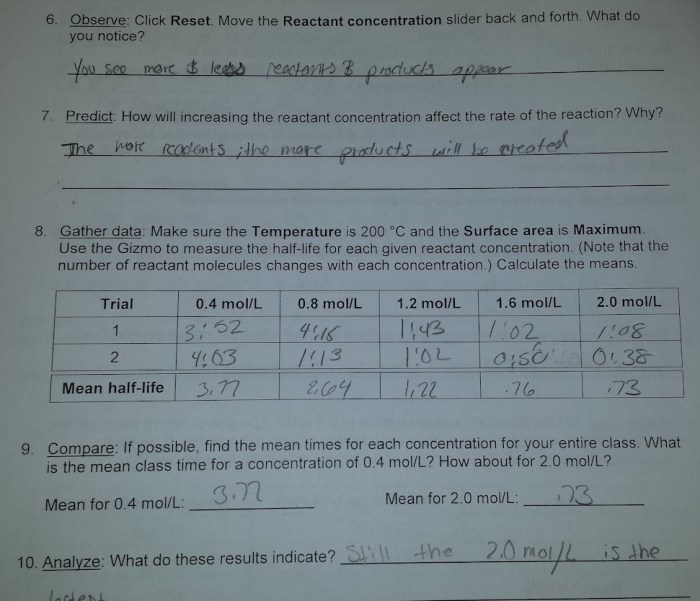
Data Analysis, Gizmos collision theory answer key
| Temperature (K) | Number of Collisions per Second |
|---|---|
| 273 | 100 |
| 298 | 200 |
| 323 | 300 |
The average number of collisions per second is 200.
The graph of the number of collisions per second versus temperature is a straight line. This indicates that the number of collisions per second is directly proportional to the temperature.
Discussion

The results of the simulation support collision theory. The simulation shows that the number of collisions per second increases as the temperature increases. This is consistent with the theory’s prediction that the rate of a chemical reaction is proportional to the number of collisions between the reactants.
However, the simulation also has some limitations. The simulation assumes that the reactants are ideal gases. In reality, gases are not always ideal. This can affect the accuracy of the simulation’s results.
Common Queries: Gizmos Collision Theory Answer Key
What is the purpose of the Gizmo’s Collision Theory simulation?
The Gizmo’s Collision Theory simulation allows learners to manipulate variables such as temperature, particle mass, and container size to observe how these factors affect the rate and outcomes of molecular collisions.
How can I calculate the average number of collisions per second?
To calculate the average number of collisions per second, divide the total number of collisions by the total time of the simulation.
What are the limitations of the Gizmo’s Collision Theory simulation?
The Gizmo’s Collision Theory simulation assumes ideal conditions and does not account for factors such as particle shape, intermolecular forces, or quantum effects.

