Unveiling the intricacies of chemistry, the Empirical Formula and Percent Composition Worksheet delves into the fundamental concepts that underpin our understanding of chemical composition. This meticulously crafted guide empowers learners with the knowledge and tools to navigate the complexities of empirical formulas and percent compositions, equipping them for success in various scientific disciplines.
Through a series of engaging activities and thought-provoking exercises, this worksheet provides a comprehensive exploration of the relationship between empirical formulas and percent compositions. It unravels the steps involved in determining empirical formulas from percent compositions and vice versa, fostering a deep understanding of these essential chemical concepts.
Empirical Formula Definition and Background

An empirical formula is a chemical formula that represents the simplest whole-number ratio of elements present in a compound. It provides the relative proportions of different atoms in a compound but does not specify the arrangement of atoms or the molecular structure.
For example, the empirical formula for glucose is CH 2O, indicating that glucose contains carbon, hydrogen, and oxygen in a 1:2:1 ratio. Empirical formulas are distinct from molecular formulas, which represent the actual number and arrangement of atoms in a molecule.
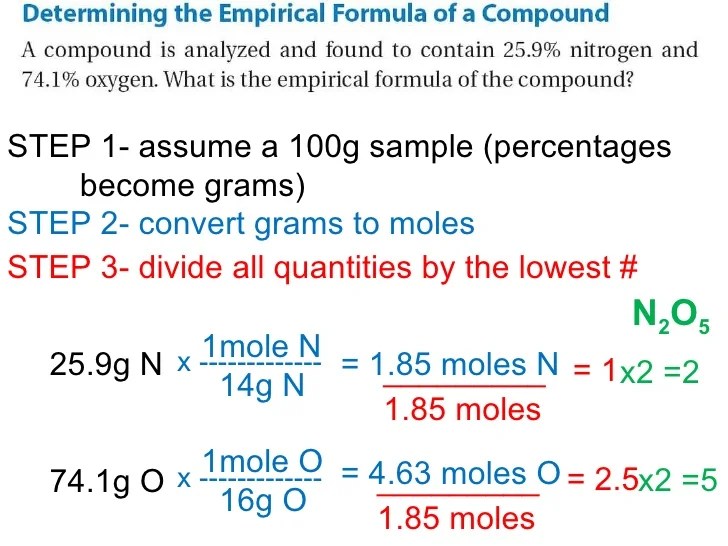
Determining Empirical Formula from Percent Composition
To determine the empirical formula of a compound from its percent composition, follow these steps:
- Convert the percent composition to grams.
- Convert the grams of each element to moles.
- Divide each mole value by the smallest mole value to obtain the simplest whole-number ratio.
For example, if a compound has a percent composition of 40% carbon, 60% hydrogen, and 0% oxygen, the empirical formula would be CH 2.
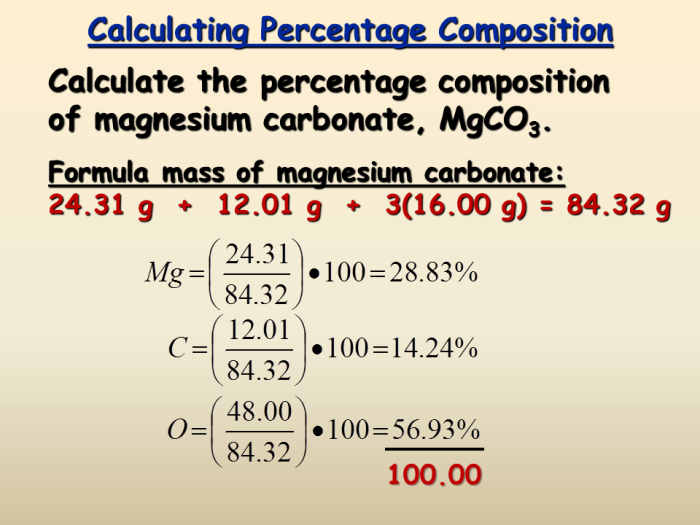
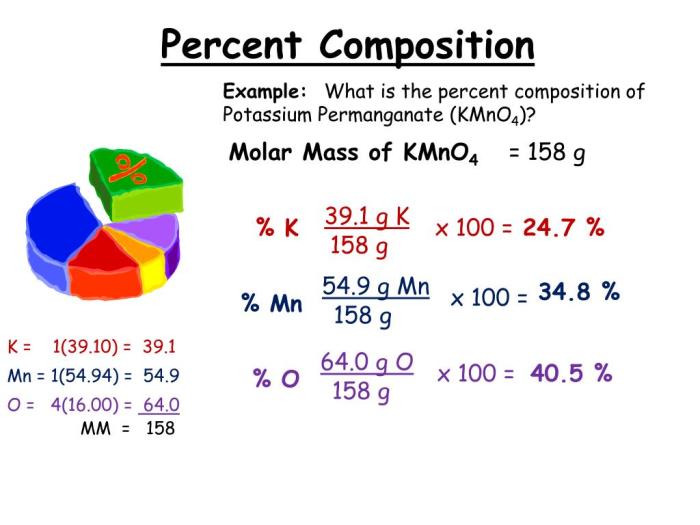
Percent Composition from Empirical Formula
To calculate the percent composition of a compound from its empirical formula, follow these steps:
- Determine the molar mass of the empirical formula.
- Calculate the mass of each element in the empirical formula.
- Divide the mass of each element by the molar mass and multiply by 100% to obtain the percent composition.
For example, the percent composition of glucose (CH 2O) is approximately 40% carbon, 6.7% hydrogen, and 53.3% oxygen.
Applications of Empirical Formula and Percent Composition, Empirical formula and percent composition worksheet
Empirical formula and percent composition are valuable tools in chemistry. They are used in various fields, including:
- Material science:To determine the composition and properties of new materials.
- Environmental science:To analyze the composition of pollutants and monitor environmental changes.
- Pharmaceutical science:To develop and optimize drug formulations.
Understanding empirical formula and percent composition helps chemists understand the chemical properties and behavior of compounds.
Worksheet Design
The worksheet should include practice problems on empirical formula and percent composition. Problems should vary in difficulty level and include a table format for students to organize their calculations.
The worksheet should also include a brief introduction to the concepts of empirical formula and percent composition, as well as a table of common elements and their atomic masses for reference.
FAQ Guide: Empirical Formula And Percent Composition Worksheet
What is the difference between an empirical formula and a molecular formula?
An empirical formula represents the simplest whole number ratio of elements in a compound, while a molecular formula indicates the exact number of atoms of each element present in a single molecule of the compound.
How do I calculate the percent composition of a compound from its empirical formula?
To calculate the percent composition, divide the mass of each element in the empirical formula by the total mass of the compound and multiply by 100.
What are some practical applications of empirical formulas and percent compositions?
Empirical formulas and percent compositions find applications in various fields, including material science, environmental science, and pharmaceutical science, aiding in understanding chemical properties and behavior.